Research Highlights
MAGNESIUM SPECIATION IN HUMAN AND RODENT TEETH
Mg K-edge XANES and EXAFS
Aug. 2, 2023
Dental enamel is a principal component of teeth, and has evolved to bear large chewing forces, resist mechanical fatigue and withstand wear over decades. Functional impairment and loss of dental enamel, caused by developmental defects or tooth decay (caries), affect human health and quality of life, with associated costs to society. Mg is an important component of dental enamel, hence, Mg molecular and atomic structural information can provide valuable information for understanding tooth development and decay.
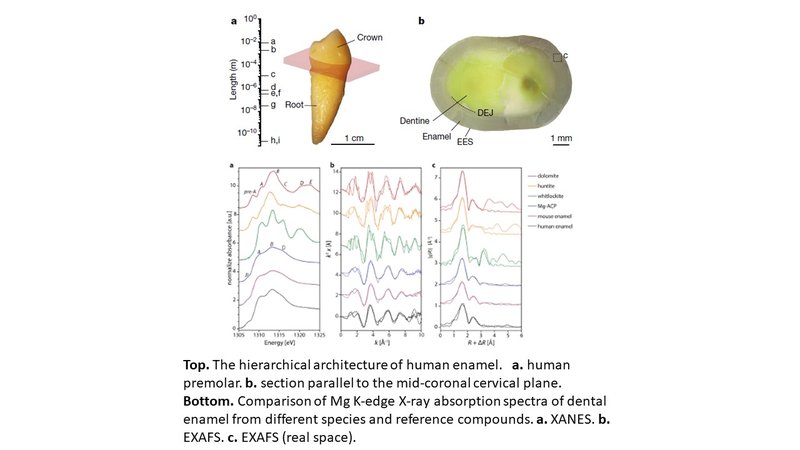
The position and intensity of transitions in Mg K-edge X-ray absorption near-edge structure (XANES) spectra are sensitive to the coordination number, geometry, bond length, and order at intermediate range. Many Mg-containing minerals can be identified by their spectral fingerprints. Spectra of rodent enamel lack the pre-A, D and E features that are characteristic for crystalline dolomite, huntite, and whitlockite. In contrast, the Mg-rich intergranular phase in rodent incisors shows a striking similarity to spectrum of synthetic Mg-substituted amorphous calcium phosphate (Mg-ACP). The dominant feature in the spectrum of synthetic Mg-ACP is the transition associated with the first coordination sphere (feature B), with little or no features that depend on order beyond the first shell. At the same time, the lower edge energy is indicative of a lower coordination number and shortrer Mg-O bond distance. The spectrum of human enamel is similar to those of rodent enamel or synthetic Mg-ACP yet display more pronounced A and B features. As these features are associated with electronic transitions in the first coordination and multiple scattering events from high shells, this indicates that a fraction of the Mg in human enamel is present in a crystalline rather than an amorphous environment, consistent with their incorporation into the crystalline core.
The Mg K-edge extended X-ray absorption fine structure (EXAFS) of human enamel is nearly indistinguishable from that of rodent enamel or synthetic Mg-ACP. In sharp contrast to the crystalline reference compounds, in which numerous scattering features from more distant shells are apparent, spectra of enamel and synthetic Mg-ACP are dominated by the nearest neighbor shell. Analysis of the local environment around Mg by fitting EXAFS spectra with theoretical scattering paths reveals that the nerarest-neighbor Mg-O bond lengths in human enamel (2.09 A), rodent enamel (2.03 A) and synthetic Mg-ACP (2.02) are notably shorter than the Ca-O bonds in OHAp (2.40 A) and ACP (2.36 A). Unlike rodent enamel, where the Mg-O bond is shorter than the Mg-O bonds in the crystalline reference compounds (2.08 to 2.11 A), that of human enamel does fall into the range. In human and rodent enamel, and Mg-ACP, this shortening is accompanied by a reduction in the coordination number from 6 to ~4, which is indicative of an amorphous material and/or presence of water in the first coordination sphere. We conclude that the environment of the majority of Mg2+ in human enamel exhibits only short- to medium-range order, with a reduction in coordination number, and the possibility of water or hydroxyl ions in the first shell, similar to the environment of Ca in ACP. However, the Mg-O shortening is not as pronounced as in rodent enamel. This is consistent with a fraction of Mg2+ occupying distorted Ca(II) sites in the apatite lattice.
DeRocher, Karen A., et al., 2020. Chemical gradients in human enamel crystallites. Nature 583: 66-71.